How polyphenols can help reduce oxidative stress and feed costs
In the last decade, a lot has been published about oxidative stress and the role of antioxidants. These antioxidants can be classified as synthetic (with added vitamin E and C) or natural polyphenols and natural vitamin E. But how do these different antioxidants work?

Oxidation processes
During normal metabolic processes, mitochondria in animal cells produce energy (ATP). This process also releases unstable free radicals as by-products. Free radicals are unstable atoms with missing electrons, making them highly reactive with other molecules. In order to become stable, free radicals take electrons from other atoms, which in turn produces other free radicals. This can lead to a so-called free radical chain reaction, causing cell damage and a loss of growth performance.
Free radicals and oxidative stress
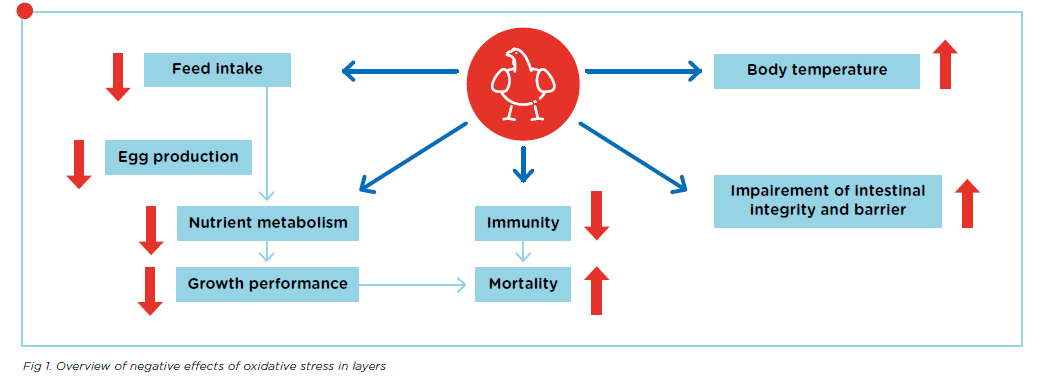
During normal metabolism, an animal’s body maintains a balance between free radicals and antioxidants which protects it from oxidative stress. Nonetheless, there are several situations that can disturb this balance, including heat or cold stress, infections, and high production levels. This oxidative stress can then lead to problems which affect animal performance, fertility, and health. Figure 1. shows an overview of these negative effects.
Neutralisation of free radicals by antioxidants
Antioxidants donate their electrons to free radicals without becoming unstable themselves. As a result, they stop the free radical chain reaction before it starts. This prevents oxidative stress so that cells stay healthy and animal performance is maintained.
One of the most important fat-soluble antioxidants is vitamin E which plays a critical role in protecting cell membranes against oxidative damage. It's just one of the many physiological antioxidants which have been identified. Others include:
- Vitamin C
- Carotenoids
- Lipoic acid
- Polyphenols
Antioxidant mechanism of polyphenols
Polyphenols are secondary plant metabolites that contain bioactive components and are characterised by multiple phenol units. They are an important group of exogenous antioxidants whose antioxidant capacity is comparable to that of vitamins E and C. Polyphenols carry a hydroxyl group which can transfer a hydrogen atom to a free radical, without the polyphenol becoming unstable. Depending on the structure of a polyphenol, it can make more than one donation to a free radical.
Regeneration of vitamins C and E by polyphenols
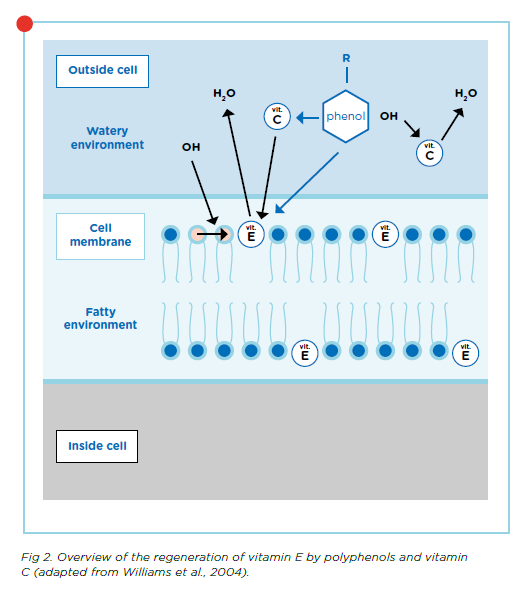
Polyphenols can also donate an electron to antioxidants that have already been used, such as vitamins E and C, allowing them to regenerate and regain their functions. Vitamin C and polyphenols are water soluble and can work inside the hydrophilic cell environment while vitamin E, which is fat-soluble, works within the hydrophobic cell membrane.
When polyphenols donate an electron to vitamin C, it can continue to scavenge free radicals and perform its other functions. In addition, vitamin C can donate an electron to vitamin E. Vitamin E can therefore continue functioning within the cell membrane without needing polyphenols to translocate into the cell to regenerate it. So even though polyphenols are not directly present in the cell membrane, their donation of electrons works both inside and outside the cell membrane as shown by the green arrows in Figure 2.
Replacing vitamin E with polyphenols
Antioxidants like vitamin E and C are commonly used in feed. Due to the regeneration of both vitamins by polyphenols and their direct antioxidative mode of action, vitamin E can partially be replaced by polyphenols. This often leads to a reduction of premix and feed costs while maintaining and sometimes even improving animal performance.
